MAIN MENU
About Diffusion Dialysis
Diffusion Dialysis Systems
Mech-Chem Associates has extensive experience with acid recovery systems including diffusion dialysis (membrane technology) systems. Our diffusion dialysis systems, which provide purified acid from waste metal, anodizing, pickling, milling and etching baths are simple to operate, dependable, and economical.
The purification systems uses an advanced separation technology known as diffusion dialysis, to separate and recover strong acids from contaminant dissolved metals. Our technology recovers nitric, hydrochloric, hydrofluoric, fluoboric, and sulfuric acids from concentrated baths that would have been discarded in the past. The acid is returned to the process for continual use, while only an acid depleted metal containing concentrate is removed for disposal.
Typically, 80-90% of the available acid is recovered in one pass through the diffusion dialysis system, with 70-90% of the dissolved metals removed from this process acid.
Each system is sized to maintain an optimum level of acid and dissolved metals in the process bath. This produces a dependable chemical process with predictable, reproducible results, part after part. Oxide removal rates and metal dissolution rates are more tightly controlled throughout the bath life for greater process control. Metal finishers and anodizers can maximize production rates without the fear of burning or loss of color uniformity, while running a cooler bath.
Acid Recycling Advantages
Acid purification and recycling using diffusion dialysis provides several direct and indirect benefits as follows:
- Process acids used indefinitely
- Improved process control and product quality
- Maintain bath uniformity for optimum performance
- Reduced waste disposal costs
- Reduced neutralization costs
- Up to 90% reduction in new acid purchases
- Functions on most commonly used acid baths
- Fully automated with low operating cost
- Simple – Reliable – Economical
- Reduced long-term liability
Applied Diffusion Dialysis
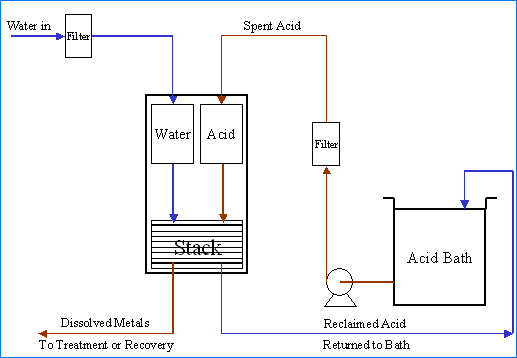
Diffusion dialysis is a membrane separation process. It has been successfully used for many years for the separation and recovery of acids from dissolved metal-bearing solutions. Diffusion is the spontaneous movement of a material from an area of high concentration to an area of lower concentration. Driven by the concentration difference, the movement of material will continue on its own until the concentration difference no longer exists. Dialysis is the separation of molecules due to the differences in the rate of movement of the molecules through a semi-permeable barrier.

In the recovery of acids with diffusion dialysis, an anion exchange membrane acts as a semi-permeable barrier placed between a flowing water stream and a flowing acid with dissolved metal solution. The anion exchange membrane has fixed positive charge located on its surface. These positive charge locations attract the negatively charged anions in solution that come in close contact with the anion exchange membrane surface.
In the case of sulfuric acid anodize baths the overwhelmingly predominant anion is the sulfate ion, SO4. As these sulfate ions in the sulfuric acid anodize solution are attracted to the membrane they are also driven by the concentration difference to diffuse across the membrane to the water side.
Simultaneously, the thermodynamic Law of Electroneutrality (in solution total charge must balance to zero) requires that the transference of every sulfate ion, which carries two negative charges, be accompanied by the transference of two positive charges.
Positively charged ions, such as Al +3 or other metal ions, are strongly inhibited from crossing the positively charged membrane because of the repulsion between like charges. The hydrogen ion, present in the acid solution as H3O +1 ions, or protonated water, is also positively charged, but is able to cross the membrane with very little hindrance. This is due, in part, to the high concentration of hydrogen ion in the acid solution and also, in part, because of the highly associated nature of water, which allows the hydrogen ion to effectively delocalize its charge.
The net effect is that the rate of diffusion of an acid across the membrane is an order of magnitude greater than that of the dissolved metal. Finally, by causing the flow of the acid solution to be in the opposite direction to the flow of water (counter-current flow), optimal advantage of the necessary concentration gradients can be realized. The results are that the water entering the diffusion dialysis system exits as a metal-depleted recovered acid solution and that the acid solution entering the diffusion dialysis system exits as an acid-depleted dissolved metal-bearing solution.
After desired results are achieved, little maintenance or no maintenance at all is required on the unit. The metering pumps are typically automatically operated 24 hours per day, seven days per week. The system has built in alarms to prevent overflowing of the liquids in the holding chambers at all times. We advise inspecting the filters and replacing them as often as necessary. If the bath has a lot of particulates it may make sense to replace the filters more often. Mech-Chem Associates offers a full line of replacement and spare parts.
The membrane life in a typical diffusion dialysis system from Mech-Chem is between 5-10 years: 5 years for strong, oxidizing acids such as nitric and hydrofluoric, up to 10 years for hydrochloric and sulfuric acids.
If the acid chemistry is the same, then one unit can be plumbed to multiple baths. It is not recommended to switch from one type of acid to another, such as hydrochloric to nitric. This is because the membrane acts like ion-exchange resin and will retain the dissolved acids on the membranes unless a special cleansing step is undertaken.
Mech-Chem Associates offers complete design, build, installation, and start-up of diffusion dialysis systems, specifically designed to meet your needs.
Diagram of Diffusion Dialysis Acid Recovery
Sulfuric Acid Anodizing
What is Sulfuric Acid Anodizing?
The Anodizing process produces an oxide film on an Aluminum part that is made the anode in an electrolytic cell. This film causes the Aluminum surface to be hard, corrosion and abrasion resistant, with excellent wear properties. Various electrolyte solutions can be employed, but the most commonly used is Sulfuric acid.
By controlling the electrolyte and the anodizing conditions, such as temperature, current density and air agitation, one can produce Aluminum coatings with almost any desired property.
Why should I use Diffusion Dialysis with my Sulfuric Acid Anodizing process?
Diffusion Dialysis is ideally suited for the recycling of sulfuric acid anodizing solutions. Diffusion Dialysis provides improved anodize quality, consistent anodized color and consistent anodic thicknesses, cooler and less energy demanding baths, while eliminating production down-time associated with the dumping and remaking of the anodize bath.
The passive, continuous Diffusion Dialysis process enables the anodizer to efficiently remove and control the dissolved aluminum content in the bath while recovering and returning a high percentage of the sulfuric acid back into the process bath. The Diffusion Dialysis process also removes and controls other contaminant build-up in the anodize bath, such as: copper, iron, lead, magnesium, manganese, phosphate, silicon and zinc, while producing a minimum of rejected waste by-product for subsequent treatment and disposal.
At anodizing job shops and captive shops across the U.S. diffusion dialysis users report improved anodize quality and reduced rework, often with reduced processing times. Additionally, dialyzed anodize baths tend to run cooler, using less energy, and thus cost less to operate.
